KetoLipix Therapeutics
Pioneering innovative ketogenic drug therapies.

Our Vision and Mission
Our vision is to treat diseases and improve patients’ quality of life based on new therapeutic concepts, shifting the frontiers of classical pharmacology and pharmaceutics.
Based on our outstanding experience in rationally designing and profiling ketone esters we will leverage unique chemical and pharmaceutical properties of our portfolio to design, develop and successfully bring to the market highly innovative drug products for a variety of indications with high medical need.
News
KetoLipix announces establishment of drug discovery pipeline
Science & Technology

Our technology aims for ketone-esters with high ketogenic load, favorable taste, predictable sustained-release, food-compatibility and optimal tolerability. With patients in mind, products easily blending into their daily routine are our aspiration.
Proprietary Chemical Core Shell Technology
KetoLipix is the leader in ketone ester technology for pharmaceutical applications, aiming at bringing to market ketone ester-based pharmaceuticals for the first time. We have developed and own the world’s largest proprietary ketone ester library to be applied as active pharmaceutical ingredients. Our portfolio is reflected by 14 patent families owned by KetoLipix. The specifically designed, core mechanistic effect of these compounds is the exogenous induction of a metabolic state of ketosis. The technology applied allows the creation of a wide range of physico-chemical properties, molecular functionality and tuning of resulting PK/PD profiles. Our available compound pool thus includes substances with fluid to highly viscous, or lipophilic to hydrophilic properties.
Safety and quality built in: Our drugs are designed using non-toxic building blocks and the proprietary manufacturing procedures were established to avoid reagents of toxicological concern. Full GMP status can be provided at scale.
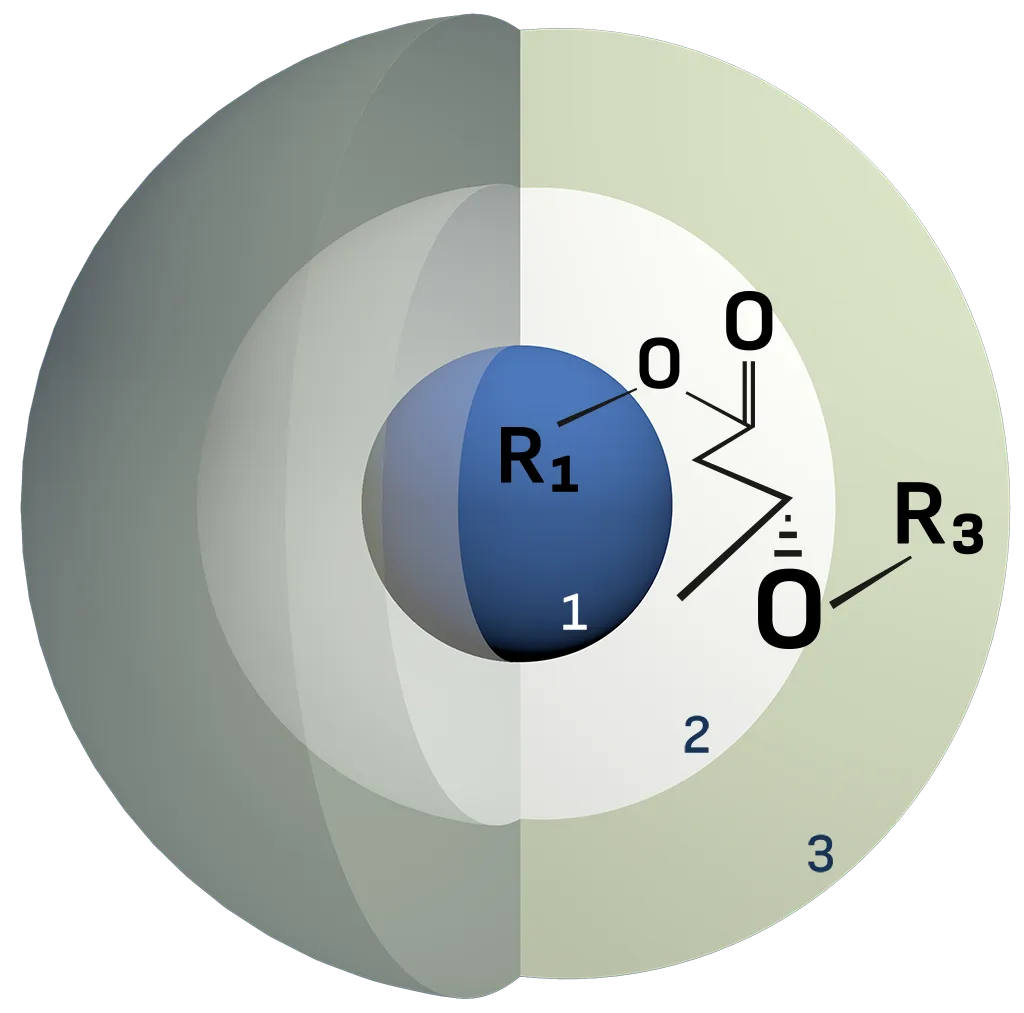
1 = Core: Non-burdening carrier / non-active
2 = Metabolic layer 1
3 = Modulation/pre-metabolite layers
Clinical and therapeutic development goals
Research on the state of ketosis and its beneficial effects in a variety of indications has been ongoing since years. KetoLipix’ objective is to provide patients benefiting from this metabolic state with an effective, safe, standardized and easy to use product to treat their disease. Therapeutic approaches have been described in various medical fields like cancer, age related muscle atrophy (sarcopenia), or for niche indications like long-chain fatty acid oxidation disorder (LC-FAOD) and the GLUT1 deficiency syndrome.
High evidence exists for the successful treatment of drug-resistant epilepsy in children by strictly abiding to a ketogenic diet (KD). KD, however, imposes a high burden on patients and families due to its organizational complexity and dietary restrictions that are especially tough for young children. There is a high medical need for a safe and effective treatment that ensures compliance and easily integrates into the life of affected children and their families. KetoLipix is positioned to exactly address these therapeutic needs.
The ketogenic and related diets in adolescents and adults – a review. Epilepsia, 52(11):1941–1948, 2011. doi: 10.1111/j.1528-1167.2011.03287.x
Optimal clinical management of children receiving dietary therapies for epilepsy. Epilepsia Open, 3(2):175–192, 2018. doi: 10.1002/epi4.12225
Ketone body metabolism and cardiometabolic implications for cognitive health. npj Metabolic Health and Disease| (2024) 2:29. doi.org/10.1038/s44324-024-00029-y
Delivery of ketone bodies through ketone esters
About us
Company
KetoLipix Therapeutics GmbH was founded as a biotech start-up in 2022 to establish a pipeline of novel therapeutic products that are based on proprietary chemical structures. 14 patent families are fully owned by the company. The company is a spin-off from IOI Oleo GmbH, Germany, and part of the IOI Group of companies, one of the leading vegetable-based oleochemical producers in the world. In the past years, the company has established a portfolio of several different classes of ketogenic compounds which are currently in development as pharmaceutical products for diseases and indications with high medical need.
Management and Team
Board
Mark Tuchen
(CEO/CFO)

Dieter Nachtigall
(COO)

Michael Beckert
(Clinical and Regulatory Consultant)

Tan Kean Hua
(Chairman)
Dirk Lochmann
(Inventor/Pharmaceutical Development)

Tanja Freichel
(Analytical Development)

Sebastian Reyer
(Inventor/Chemical Development)

Jason Rushton
(Board Member)

Management and Team
Mark Tuchen
(CEO/CFO)

Dieter Nachtigall
(COO)

Michael Beckert
(Medical Consultant)

Dirk Lochmann
(Inventor/Pharmaceutical Development)


Tanja Freichel
(Analytical Development)

Sebastian Reyer
(Inventor/Chemical Development)
Board
Tan Kean Hua
(Chairman)

Jason Rushton
(Board Member)


Mark Tuchen studied Business Administration before starting his professional career in 2000 in a Big 4 audit and consulting company where he served a wide range of customers as senior manager for more than a decade. In 2013 Mark became Chief Financial Officer of todays’ IOI Oleo GmbH, Germany, where he took over responsibility for all Finance & Legal related matters, HR, IT and later Procurement. As CFO, Mark is a Member of the Management Board of IOI Oleo GmbH. He accompanied the foundation process of KetoLipix Therapeutics GmbH in 2022 and served as Board Member before being elected as CEO in 2024.

Dieter Nachtigall has more than 25 years of professional experience in global drug discovery and development along the whole value chain from translational sciences / discovery to approval of the new drugs. He held several senior and executive management positions both in large pharma and biotech with a focus on R&D project management, NCE and NBE analytical development, and global quality management. Before joining KetoLipix he was CEO of QUANTRO Therapeutics in Vienna. Dieter is a chemist by training and has a PhD in analytical chemistry.

Michael Beckert, physician by training and former clinician at the Charité Berlin, has more than 25 years of experience in big pharma as well as small biotech start-up companies. Starting his pharma career at MSD Germany, he subsequently held positions as Chief Medical Officer at Scil Biomedicals, Germany; Lifecycle Pharma Denmark/USA; and Ascendis Pharma in Denmark. In these roles he was leading preclinical, clinical and regulatory affairs and being responsible for the approval of several products in the US and Europe. Since 2009 Michael also works as a Consultant to Biotech Companies accompanying start-ups from inception to exit.

Dirk Lochmann is a pharmacist by training and holds a PhD in pharmaceutics. He has been working in pharmaceutical research and industry in various positions for more than 20 years and is a core member of the inventor team of the KLX ketogenic API library.

Tanja Freichel studied chemistry and completed her doctorate in the field of synthetic biomimetics. In 2019 she joined the R&D Team of the IOI Oleo GmbH as a research chemist focusing on the synthetical and analytical development of the KLX ketogenic API library. Currently she is the Head of the Synthesis Laboratory and Pilot Plants in the IOI Oleo R&D department, working on the implementation and scale-up of new products and technologies.

Sebastian Reyer is a chemist and holds a PhD in organic and industrial chemistry. He has been working in the chemical industry in manufacturing and R&D for more than 15 years in various positions. Currently he is the Director of Science & Innovation and Member of the Management Board of IOI Oleo GmbH, Germany. Sebastian is a core member of the inventor team of the KLX ketogenic API library.

Jason is an accomplished life sciences investor with over three decades of experience within the sector including drug development, drug discovery, management consulting, venture capital, strategy consulting and corporate finance advisory.
Jason joined Xeraya Capital from Deloitte Geneva, Switzerland, where for five years he was the Director, Corporate Finance Advisory, leading the firm’s healthcare and life sciences advisory (M&A) business.
His working career in the venture capital industry began with the Merlin Biosciences Fund and later with the Inventages (Nestlé) Fund where he sourced, evaluated and closed numerous venture capital investments and was involved in post investment management of portfolio companies, including taking on board positions. Prior to that Jason was a management consultant in the Life Sciences Group of PA Consulting. His early career was in the sciences in drug discovery with Eli Lilly.
Jason is a Board member of several life science companies and holds a Master of Science degree in Immunology, and a Bachelor of Science (Hons) degree in Anatomy, both from the University of Birmingham, UK.

Mr Tan Kean Hua held a senior marketing position in an oleochemicals multinational company prior to joining IOI Group in 2004. He was the Chairman of the Malaysian Oleochemical Manufacturers Group from March 2010 to March 2017, during which period he also held the chair of the ASEAN Oleochemical Manufacturers Group twice. He was a member of the Board of MPOB for three (3) terms from May 2010 to May 2017. Previously, he was the Executive Director of IOI Oleochemical Division before being promoted to Deputy Group Chief Executive Officer on 1 July 2024.
